 Information
Information
Symbols - The following symbols may be used where applicable in labeling for LGC Clinical Diagnostics products:
 Lot Number
Lot Number
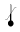 Temperature Limit
Temperature Limit
 Biological Risk
Biological Risk
 Caution
Caution
 Expiration Date
Expiration Date
 Instructions for Use
Instructions for Use
 In Vitro Diagnostic Medical Device
In Vitro Diagnostic Medical Device
 Manufacturer
Manufacturer
 Catalog Number
Catalog Number
 Do Not Reuse
Do Not Reuse
Most products are registered in the EU and U.K. For availability in these and other countries, check with your local distributor or contact cdx-sales@lgcgroup.com